Data/Graphs Procedures Baking Soda and Vinegar Reaction Step 7 through 8 Step 7 slowly put the end of the balloon to the top of the bottle with vinegar DO NOT PUT BAKING SODA IN BOTTLE YET!That lift is the gas produced from the two ingredients is carbon dioxide or CO2With school out for summer we've been busy doing all kinds of outdoor learning activities!One of our favorites is this baking soda

Wait Weight Don T Tell Me Chemistry Earth Science Science Activity Exploratorium Teacher Institute Project
Vinegar and baking soda balloon experiment observation
Vinegar and baking soda balloon experiment observation- Best Answer Copy The most basic observation would be that mixing baking soda and vinegar causes a chemical reaction the results in part of the solution becoming a gas Wiki User ∙Baking soda Chemical name, sodium bicarbonate with formula NaHCO 3 Vinegar A dilute solution of acetic acid in water Acetic acid is also known as ethanoic acid, with the formula CH 3 COOH A beaker or jar The chemical reaction When baking soda is mixed with vinegar, something new is formed The mixture quickly foams up with carbon




Baking Soda Vinegar Balloon Experiment Easy Fun Kid Friendly Things To Do
Henry was getting a kick out of the experiment and loved watching it overflow the cupMix everything together and watch as the reaction creates carbon dioxide and inflates the balloons!Step 8 put the the baking soda in bottle FAST!
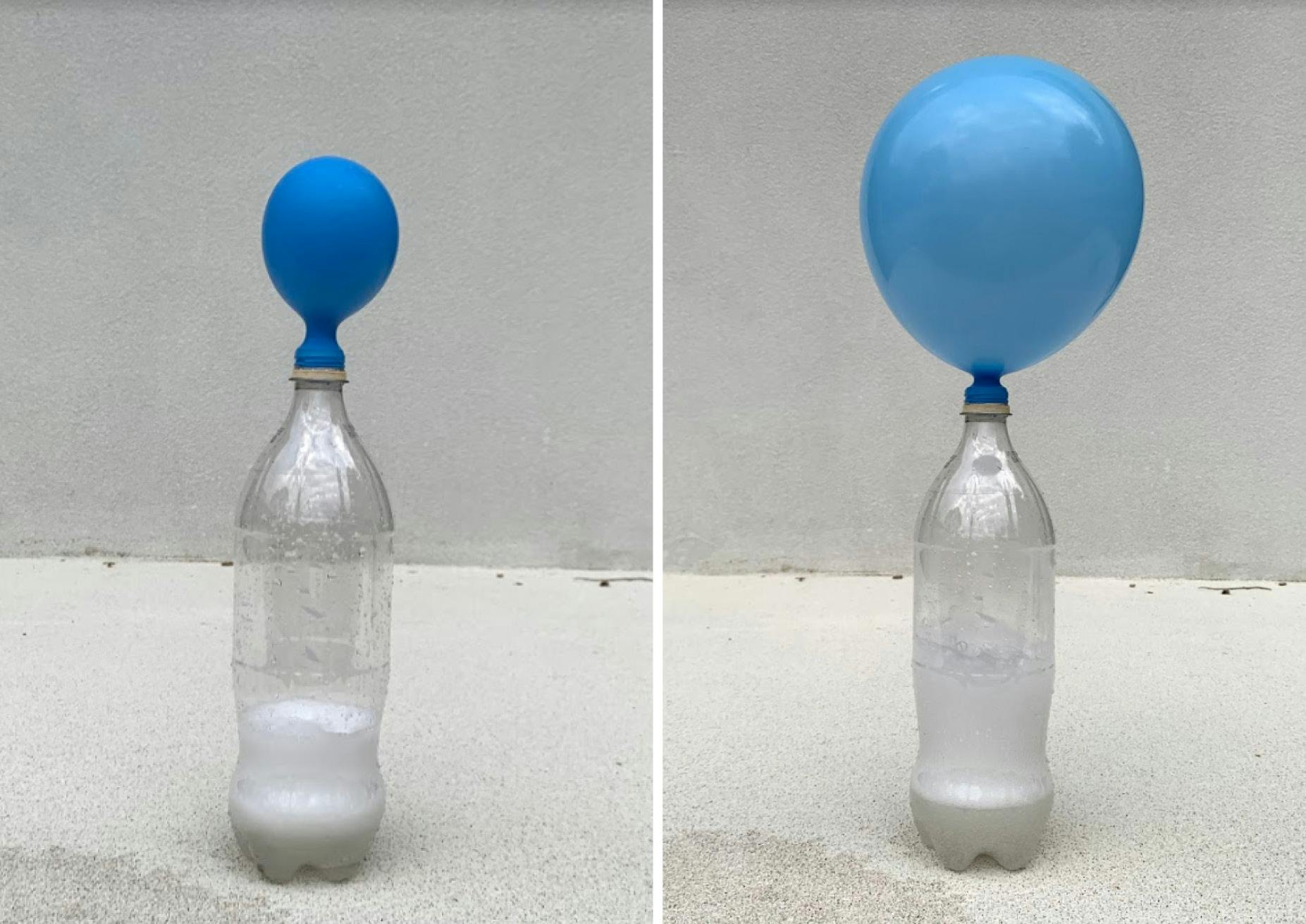
Repeat for each type of vinegar Measure the circumference ofThat lift is the gas produced from the two ingredients is carbon dioxide or CO2 Using baking soda bicarbonate, have kids hold a funnel while you pour about 2 cups into a 2 liter bottle Then pour about 1 cup of vinegar using the same funnel and quickly take out Immediately put a balloon on top of the 2 liter bottle and watch it inflate!!
3 Stretch the open end of the balloon over the neck of the bottle Make sure it's on tight!Adding baking soda to vinegar, the reaction is delayed, but then fizzes the same amount More vinegar is better A 12 to 1 ratio of vinegar to baking soda caused a fizzing explosion!As far as science experiments go, this is a pretty simple one I love that we had all of the materials needed for it at home already, and that it was quick and easy to put together!




What S The Difference Between Baking Soda And Baking Powder American Chemical Society
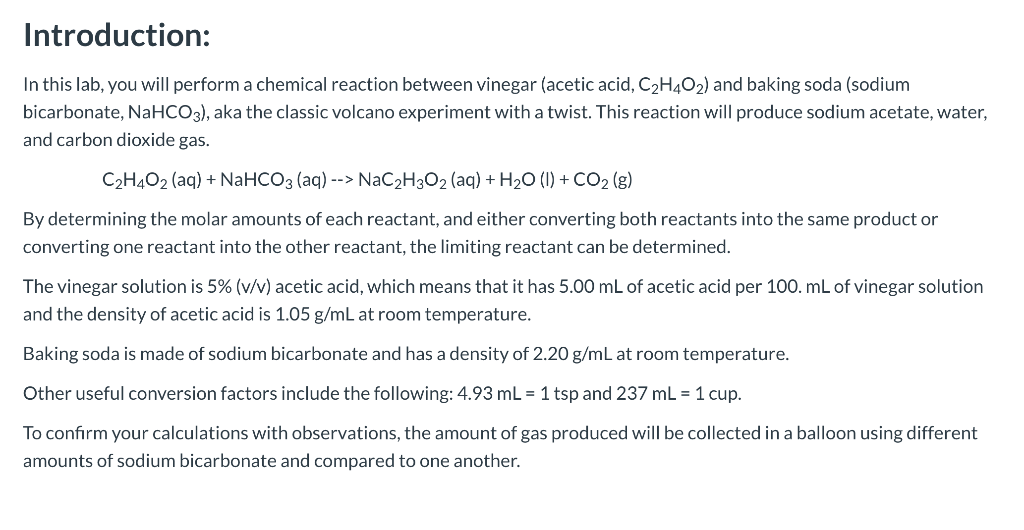



Solved Introduction In This Lab You Will Perform A Chegg Com
The baking soda/vinegar balloons is a fascinating demonstration of acid base chemistry Vinegar is water with about 3 percent of a chemical called Acetic acid Baking Soda is a compound called Sodium Bicarbonate, also known as Sodium Hydrogen Carbonate (NaHCO 3), and is a base So the reaction occursAfter three minutes place the balloon on top of the bottles, being careful not to spill the baking soda into the bottles yet Set your timer Tip both balloons upwards to drop the baking soda into the vinegar Record your results Results Baking soda and vinegar should have been able to blow up the ballon every timePour the vinegar into the bottle Carefully fit the balloon over the bottle opening (be careful not to drop the baking soda into the vinegar yet) Once the balloon is fitted snugly on the nozzle, hold up the balloon and allow the baking soda to fall into the vinegar Observe the chemical reaction and effect on the balloon




Balloon Blow Up Science Experiment Children S Museum Of Sonoma County




Blow Up A Balloon Using Science Laughing Kids Learn
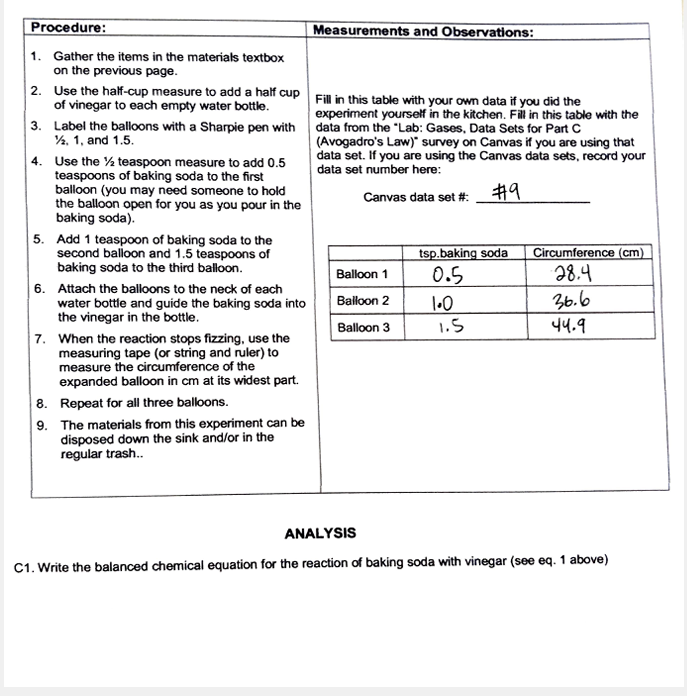
Balloon STEM Activity Like our bottle rockets, in this activity we are capturing the CO2 gases that result from a baking soda and vinegar reaction Using our STEM skills we tested different ratios to see how it affects the inflating of our balloons We have done this experiment for Groundhog Day and HalloweenBalloon 1 05 284 6 Attach the balloons to the neck of each water bottle and guide the baking soda into Balloon 2 10 366 the vinegar in the bottle Balloon 3 15 449 7 When the reaction stops fizzing, use the measuring tape (or string and ruler) to measure the circumference of the expanded balloon in cm at its widest part 8 Procedure Use exactly 50 grams of baking soda in each balloon that you test Only vary the amount of vinegar Technique put baking soda into a balloon Put vinegar in a test tube Stretch the balloon over the top of the test tube and pour the vinegar into the balloon Put the test tube, with the balloon on it, into a rack and observe




Balloon Baking Soda Vinegar Kids Science




Classroom Resources Inflating A Balloon With Chemistry ct
#floating balloon#Inflating Balloon#shorts#Soda & Vinegar #balloon seller#air pump hot air#DIY science with eno #eno reaction with balloon#eno reaction withThe science, behind this balloon baking soda experiment, is the chemical reaction between the base {baking soda} and the acid {vinegar} When the two ingredients mix together the balloon baking soda experiment gets it's lift!We could have kept going with this all afternoon!
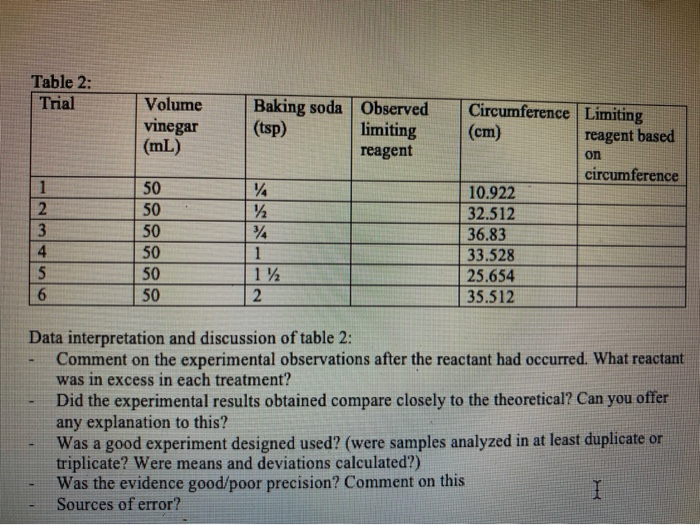



This Experiment Involved Putting 50ml Of Vinegar In A Chegg Com




Vinegar And Baking Soda Balloon Experiment Happy Brown House
Using the funnel, put the baking soda into the balloon Pour the vinegar in the bottle Now attach the balloon to the opening of the balloon in such a way that it fits closely, without leaving any gap Hold the balloon up so that the baking soda falls into the vinegarBaking soda is bicarbonate (NaHCO3) and vinegar is acetic acid (HCH3COO) One of the products this reaction creates is carbon dioxide, which makes the bubbles When the baking soda meets the vinegar, there is a chemical reaction as carbon dioxide gas is created and fills the balloon causing it to inflate Carbon dioxide is an important gas inHere's a super cool science experiment for kids that requires only a few common materials In this experiment you'll create a chemical reaction using baking soda and vinegar that will make a baggie explode!




Balloon Baking Soda Vinegar Science Experiment For Kids




How To Inflate Balloon With Vinegar And Baking Soda Science4fun
Spoon TIP Before starting the experiment, you will want to stretch out the balloon to make it more loose and easier to inflate Step 1 Pour 12 spoonfuls of baking soda into the opening of the balloon, using a funnel You'll need to shake it a bit to get it down into the base of the balloon Step 2 Use the funnel again and pour some The reaction between baking soda and vinegar actually occurs in two steps, but the overall process can be summarized by the following word equation baking soda ( sodium bicarbonate) plus vinegar (acetic acid) yields carbon dioxide plus water plus sodium ion plus acetate ion The chemical equation for the overall reaction is with s = solid, l These baking soda and vinegar balloons were so much fun!




Baking Soda And Vinegar Reaction Earth Day Science Experiment




Science For Kids Chemical Reactions Using Baking Soda And Vinegar Buggy And Buddy
Chemical reaction science experiments using baking soda and vinegar are a lot of fun and are great learning opportunities In this quick and easy experiment, we are going to use an endothermic chemical reaction and the resulting carbon dioxide caused by mixing baking soda and vinegar to inflate a balloon Materials Empty plastic or glass bottle Balloon 1 cupRead MoreAfter Mixing Mass altogether of Erlenmeyer flask, vinegar, balloon, and baking soda 142 Grams Observations of reaction and products A chemical change has occurred with the mix of vinegar and baking soda Water, carbon dioxide and sodium acetate are produced Vinegar and baking soda foam up when mixed and create a gas which fills the balloon 1Let the heavy end of the balloon dangle, so no baking soda goes in the bottle 4 Hold onto the balloon at the bottle neck, and pick up the heavy part of the balloon so that all the baking soda falls into the vinegar at the bottom of the bottle 5




Baking Soda Vinegar Balloon Experiment Easy Fun Kid Friendly Things To Do



Science Lab Science Isn
//mocomicom/ presents Baking Soda, Vinegar and Inflating Balloon Blow Up Science Experiment for KidsREQUIREMENTS BalloonVinegarBaking SodaBottleWhen you added the baking soda to the vinegar, the two combined to make carbondioxide gas, which inflated the balloon The expansion of the balloon changed the weight of your sealed flask because you and your entire experiment are submerged in a fluid air Just like water, air is a fluid, and fluids buoy up objects This experiment demonstrates how states of matter can change – mixing a solid with a liquid to create gas!




Wait Weight Don T Tell Me Chemistry Earth Science Science Activity Exploratorium Teacher Institute Project




Self Inflating Balloon Baking Soda And Vinegar Balloon Experiment Teach Beside Me
The science, behind this balloon baking soda experiment, is the chemical reaction between the base {baking soda} and the acid {vinegar} When the two ingredients mix together the balloon baking soda experiment gets it's lift!The science, behind this balloon baking soda experiment, is the chemical reaction between the base – baking soda – and the acid – vinegar When the two ingredients mix together the balloon baking soda experiment gets its lift!Steps to this experiment1 Put some vinegar in an empty water bottle Fill less than half of the water bottle You don't want too much 2 Use a funnel to put baking soda in a balloon3 Stretch the balloon over the top of the water bottle without allowing any of the baking soda




Science The Scientific Method Lesson 3 The Scientific Method Balloon Experiment Ppt Download




Balloon Baking Soda Vinegar Experiment For Kids Bilingual Education Activities
In this experiment carbon dioxide produced in the reaction inflated the balloon The baking soda and vinegar fizzed out and bubbles were produced, and the flask was cool at that time So, these observations told us that the chemical reaction occurred The reaction stopped when we run out of the reactants that were baking soda and vinegar WhenThis experiment introduces children to Properties of Matter, and Cause and Effect, two key science principles When baking soda and vinegar are combined, a gas called Carbon Dioxide is created which then inflates the balloon 3 Stretch the neck of the balloon over the top of the bottle Be careful not to spill the baking soda while you do this Hold the balloon's neck with both hands and stretch it over the top of the plastic bottle containing vinegar Have a friend keep the bottle steady if the table or bottle is wobbly




Teacher Guide




Acid Rain Chemical Reactions Acid Rain
Conclusion The experiment and result of it supported our hypothesis that the bubbles would float on top of the mixture of the baking soda and vinegar It did this because when we combined the baking soda and vinegar it had a chemical reaction that produces carbon dioxide gas This layer of pure carbon dioxide was more dense than the bubble Simple Science Baking Soda Vinegar Balloon Experiment Balloon Science Experiments Easy Science Fair Projects Science Experiments Kids Preschool NaHCO2 is an amphoteric compound this means that it would react as an acid and a base Topic Measure the mass of a beaker 10mL vinegar and 10g baking soda Simple Science Baking Soda Vinegar BalloonCreating a reaction between baking soda and vinegar is a classic science experiment that kids of all ages love to watch We've actually used the mixture in a few different science experiments ourselves including our green themed experiment and our soda bottle speed boats But adding a balloon to the mix just ups the fun factor and makes it seem like a brand new experiment all



Koch Newsroom Baking Soda Balloons




Balloon Baking Soda Vinegar Experiment For Kids Bilingual Education Activities
Scientific Method Hypothesis My hypothesis is that the acid and chemicals in vinegar (acetic acid) and the chemicals in baking soda (Sodium bicarbonate) when mixed will cause a reaction that will inflate the balloon Conclusion/Results After doing my experiment, my results were that when vinegar mixes with baking soda the reaction will cause the gas particles to inflate the balloonStep 1 Pour your vinegar into the bottle Set aside Step 2 Use your funnel and slowly measure in your two cups of baking soda Step 3 Take the bottle ring out of your bottle opening and add it over the balloon opening Step 4 Then snugly close the bottle opening with the balloon and add the ring back to secure in place1 Fill the bottle halfway with vinegar 2 Use the funnel to put two teaspoons of baking soda in the balloon 3 Carefully stretch the balloon over the bottle 4 Shake the balloon so all the baking soda goes in and watch the balloon inflate




Baking Soda And Vinegar Balloon Experiment Youtube




Designing An Experiment Using Baking Soda And Vinegar Pdf Free Download
This experiment is investigating if baking soda and vinegar will inflate a balloon Baking soda is a chemical salt that occurs in its natural form as the mineral NaHCO2 NaHCO2 is an amphoteric compound, this means that it would react as an acid and a base1 Describe what happens when the vinegar was poured into the cup of baking soda Answers may vary, but students should mention release of a gas This is a typical chemical reaction in which an acid vinegar, reacts with a base baking soda, to produce a new chemical a salt 2 The gas produced in this reaction can put out firesThe teacher and/or students will perform the vinegar, baking soda and balloon experiment This demonstrates all three states of matter Students predict on the front and record their observations on the back




Baking Soda Vinegar Balloon Experiment The Go To List




Law Of Conservation Of Mass Lab Purpose
And on top of that, it wasBaking soda to the soda bottle This will allow you to measure the volume of air in the soda bottle The volume of gas produced by the baking sodavinegar reaction is equal to the volume of gas measured with the reaction minus the volume of gas measured without the reaction 6 Repeat steps 310 with ½ tsp of baking soda Balloons Funnels Measuring spoon Start by putting the funnel into the balloon This will make it much easier to get the baking soda inside Add baking soda We added about 4 teaspoons to each balloon To get your balloons a bit bigger than ours, you could add 1 or 2 teaspoons more Next, use a funnel to pour some vinegar into your bottles
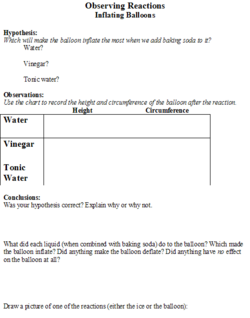



Observing Reactions Wikieducator




19 Unit 2 Matter Test Interactive Worksheet By Philippe Menjoulet Wizer Me
Pour the vinegar into the bottle carefully fit the balloon over the bottle opening (be careful not to drop the baking soda into the vinegar yet) once the balloon is fitted snugly on the nozzle, hold up the balloon and allow the baking soda to fall into the vinegar observe the chemical reaction and effect on the balloon record observationsFollow our Science for Kids board!Baking soda and vinegar balloon experiment explanation The reason that we are able to inflate a ballon in this experiment is thanks to the magical reaction of the baking soda and vinegar As the baking soda and vinegar interact they release a gas (carbon dioxide) and increase the




Vinegar And Baking Soda Balloon Experiment Happy Brown House




Science The Scientific Method Lesson 3 The Scientific Method Balloon Experiment Ppt Download
Measure 45 ml of vinegar and pour it into a water bottle Put the mouth of the balloon on the wine spout to keep the baking soda in the balloon (The balloon will be flopped to one side) Lift the balloon up and pour the baking soda into the bottle of vinegar Observe for 1 minute;Magic Balloon Recording Printables (vinegar and Baking soda) Experiment prediction and observation recording sheet (different templates to suit developmental levels) Magic Balloon Experiment science journal title print these science journal tiles onto coloured paper and use as a science journal writing prompt)Kids love learning new things, especially when the learning is paired with something




Baking Soda And Vinegar Balloons




Chemical Reactions Lab
How/why does the balloon blow up with only baking soda and vinegar?



1



5th Six Weeks Isn 12 13



Baking Soda And Vinegar Balloon Experiment Science Stock Vector Illustration Of Background School




Vinegar And Baking Soda Experiment Lesson Plans Worksheets




Baking Soda And Vinegar S Reaction Perkins Elearning




Baking Soda Vinegar Balloon Experiment Youtube




Law Of Conservation Of Mass Lab




How To Inflate Balloon With Vinegar And Baking Soda Science4fun




Balloon Blow Up Science Experiment




Vinegar And Baking Soda Experiment Sheet By Miss K Makes It All Tpt




The Teacher And Or Students Will Perform The Vinegar Baking Soda And Balloon Experiment Chemistry Experiments Science Chemistry Homeschool Science Experiments




Baking Soda And Vinegar Worksheet Teachers Pay Teachers




Balloon Baking Soda Vinegar Science Experiment For Kids




Baking Soda And Vinegar Balloons



Baking Soda Vinegar Balloon Experiment Capturing Parenthood




Creating A Science Project Elmersscienceready Detroitmommies Com




Baking Soda And Vinegar Experiment Report




Balloon Magic Science Activities For Kids Fun Science Science Activities




Science Project How To Inflate Up A Balloon With Liquid Ppt Video Online Download




Giant Balloon Baking Soda And Vinegar Experiment Hello Wonderful




Experimental Report Heavy Balloons Pptx Powerpoint




Vinegar And Baking Soda Balloon Experiment Happy Brown House




Baking Soda And Vinegar Lab Report Docx Baking Soda And Vinegar Lab Report Research Question What Is The Effect That Baking Soda Has On The Volume Of Course Hero



Magic Balloons Science Project Science Project Ideas




How To Make A Heavy Balloon By Pakalana



1




Vinegar And Baking Soda Balloon Experiment Happy Brown House




Baking Soda And Vinegar Balloon Experiment Science Project Education Com




Giant Balloon Baking Soda And Vinegar Experiment Hello Wonderful




Balloon Baking Soda And Vinegar Experiment Data Collection Sheet




Designing An Experiment Using Baking Soda And Vinegar Pdf Free Download




Balloon Baking Soda Vinegar Kids Science




Giant Balloon Baking Soda And Vinegar Experiment Hello Wonderful




Baking Soda And Vinegar Balloon Experiment




Balloon Baking Soda Vinegar Experiment For Kids Bilingual Education Activities



Balloon Experiment Learncreatelove




Science Fair Experiment Carbon Chemistry Dioxide En En En Experiments Science Soda Vinegar Glogster Edu Interactive Multimedia Posters




Self Inflating Balloon Baking Soda And Vinegar Balloon Experiment Teach Beside Me



Solved Procedure Measurements And Observations 1 Gather Chegg Com




Science Project How To Inflate Up A Balloon With Liquid Ppt Video Online Download



1




How To Fill A Balloon With Baking Soda Vinegar 5 Steps With Pictures Instructables




What Happens When You Mix Vinegar And Baking Soda Wonderopolis




Giant Balloon Baking Soda And Vinegar Experiment Hello Wonderful




Is This A Chemical Or Physical Reaction




Fun And Easy Science In The Classroom Guest Blog Post The Applicious Teacher




At Home Lesson Breathless Balloons With Edie S Experiments Penguin Books Australia




Inflate A Balloon With Baking Soda And Vinegar Pbs Kids For Parents



Baking Soda And Vinegar Worksheet Teachers Pay Teachers




Balloon Baking Soda And Vinegar Experiment Data Collection Sheet




Balloon Blow Up Science Experiment




Baking Soda And Vinegar Balloons




Baking Soda And Vinegar Lab Report Buy Essay Online Dissertationgratuite Web Fc2 Com




Science Project How To Inflate Up A Balloon With Liquid Ppt Video Online Download




Baking Soda And Vinegar Balloons




Let S Play With Reactions




Science Club Reaction Of Vinegar With Bicarbonate Of Soda



At Home Lesson Breathless Balloons With Edie S Experiments Penguin Books Australia




Balloon Blow Up Science Experiment




Baking Soda And Vinegar Worksheet Teachers Pay Teachers




Self Inflating Balloon Baking Soda And Vinegar Balloon Experiment Balloon Experiment Balloon Science Experiments Baking Soda Experiments




Baking Soda And Vinegar Balloon Experiment Science Projects For Kids Educational Videos By Mocomi Youtube




Expanding Balloon Room Ppt Download




Balloon Baking Soda Vinegar Science Experiment For Kids




Gas Balloon City Utilities




Balloon Baking Soda Vinegar Science Experiment For Kids




Baking Soda And Vinegar Lesson Plans Worksheets




Use Vinegar And Baking Soda To Blow Up A Balloon Discovery Express




Balloon Baking Soda Vinegar Experiment For Kids Bilingual Education Activities




Vinegar And Baking Soda Balloon Activity Education Com




States Of Matter Gases Lab Sheet Baking Soda Vinegar Balloon



1



0 件のコメント:
コメントを投稿